- This topic has 58 replies, 25 voices, and was last updated 10 years ago by ernie_lynch.
-
Where's all the CO2
-
ernie_lynchFree MemberPosted 10 years ago
Careful how you open that can of coke. Only do it in a well ventilated area. And ffs don’t drink it until it’s flat 😉
DracFull MemberPosted 10 years agoIf anyone doubts the danger of CO2, try breathing a mixture of air & CO2, the reflex to get away from it’ll knock you backwards.
I’m doing it now.
mogrimFull MemberPosted 10 years agoAll I can find is references to carbon monoxide being used for badger culling, not carbon dioxide. Obviously if you pump any gas other than oxygen, even nitrogen, you will eventually cause death due to oxygen displacement.
I can see certain advantages to using CO2: it’s heavier than air (apart from the CO2 bit of it, of course 🙂 ) which means it’ll sink into the sett rather than floating out; it’s also basically harmless outdoors, unlike carbon monoxide… of course, you’ll be suffocating the badgers, which is hardly the most humane way to cull them.
anagallis_arvensisFull MemberPosted 10 years agoAlthough you can breath air with oxygen removed without a care in the world as long as CO2 doesnt build up. But only for a short time.
slowoldgitFree MemberPosted 10 years agoI’d like to hear more about the dinosaur mass deaths resulting from volcanic CO2, if anyone knows details.
And do L remember right: CO2 dissolves in the blood, bicarb or whatever, CO borks the haemoglobin?
boxfishFree MemberPosted 10 years agoJust imagine if all the guff spouted on STW was actually spoken! The CO2 footprint would be enough to doom us all thrice over.
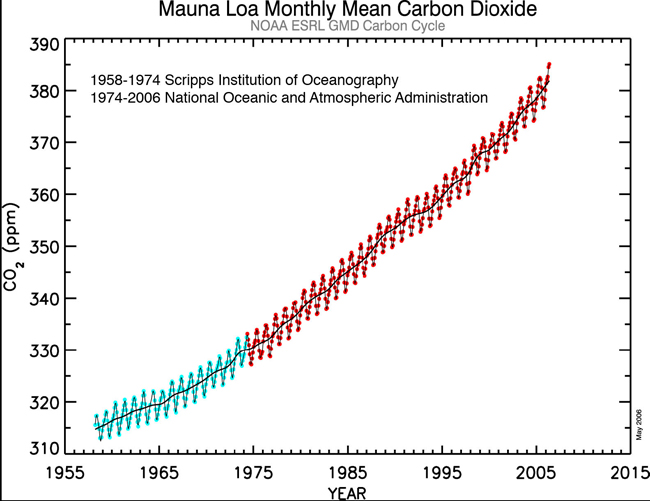
gwaelodFree MemberPosted 10 years agosurprised no one has posted the Mauna Kea graph yet showing actual real atmospheric CO2 measurements – the annual variation is caused by differnece between winter and summer co2 emissions by the biosphere in (most plant Biomass is in the northern hemisphere – much less in the southern)
Looking further back over the last 300 years or so we have this as a record of atmospheric CO2 obtained by measuring CO2 concentrations in bubbles frozen in Ice at the polar regions – each year a layer of fresh snow traps air bubbles which acts as a record of the atmospheric CO2 for that year – The mauna kea direct measurements are the thicker black lines at the right hand of the graph.
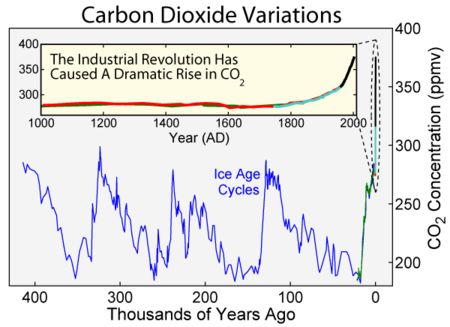
Looking back over the period of the last few ice ages we can use the Ice core records to show the change in CO2 levels in the atmosphere – again by looking at bubbles trapped in ice. –
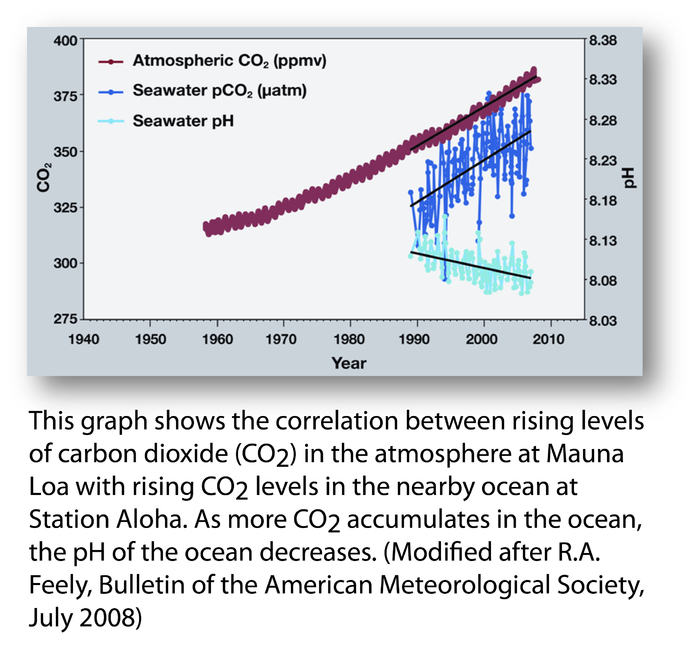
generally Ice ages tend to see CO2 levels of 200ppm, and warm bits between ice ages about 300ppm. Effectively the current atmosphere is nearly 400ppm the difference between the normal 300ppm and current 400ppm is due to human fssil fuel burning – although another big slug of CO2 has also found it’s way into the oceans as well in recent years – ( a tiny amount compared with the size and chemical composition of the oceans, but enough to shift the alkalinity slightly)
Bottom line is these shifts in CO2 levels in the atmosphere are nowhere near enough to cause toxicity problems to humans, but are enough to cause big changes in the amount of solar radiation the earths atmosphere traps, and hence the earths temperature.
gwaelodFree MemberPosted 10 years agoslowoldgit – Member
I’d like to hear more about the dinosaur mass deaths resulting from volcanic CO2, if anyone knows details.Do you mean global extinction events caused by CO2 poisoning (as far as I am aware there are none – and if CO2 levels ever got high enough to cause that then the rapid climate shift would already have caused ecosystem collapse and there would be very little left alive for the co2 to poison) or just local killings caused by eg volcanic lake outgassing. Difficult to envisage a classic volcano causing mass deaths by CO2 directly, as the rest of the volcanic eruption type event would almost certainly be more important.
Or do you mean the role that volcanic SO2 played in the Permian mass extinction – not dinosaurs per se – but probably the biggest Mass extiction event so far.
ernie_lynchFree MemberPosted 10 years agobut enough to shift the alkalinity slightly
That’s what I find particularly scarey and depressing, the thought of the widespread destruction of the natural equilibrium which occurs in the oceans, and the devastating consequences it will have to, for example, coral reefs and their inhabitants.
**** up the atmosphere is tragic enough but **** up the oceans which are so finely tuned without any slack, is too depressing to even think about imo.
gwaelodFree MemberPosted 10 years agoOn a general note terms like “co2 levels don’t need to rise a lot” is subjective and people working in one branch of science may have very different ideas of what “a lot” is. In this case physiologists and earth scientists, just be careful when using the same language in different fields.
The classic case of this subjective language is geologists using the word “recent” – depending on what sort of geologist you are talking to it could mean anytime in the last 2 Billion years, although an Archeologist would probaly be thinking in terms of 100s of years.
(although archeology isn’t proper science 😉 )
slowoldgitFree MemberPosted 10 years agoThanks gwaelod.
No, it was this bit…
Also CO2 is heavier than air – there are loads of mass dinosaur fossil sites near volcanic areas where CO2 pumped out has suffocated them,
… that caught my attention, can’t see how I missed it when it was first published. Not really my subject, but something that significant, etc.
slowoldgitFree MemberPosted 10 years agoSo if the CO2 that’s dissolving in the oceans causes anoxic conditions in the deep oceans, we’re stuffed. Not yet obviously, but we’ll get to see an Ocean Anoxic Event for real.
ernie_lynchFree MemberPosted 10 years agoI don’t think it’s lack of oxygen that will be the catastrophe, long before that the pH will have crashed. The oceans just won’t be able to cope with that. At least life within the oceans won’t.
gwaelodFree MemberPosted 10 years agoNo, it was this bit…
Also CO2 is heavier than air – there are loads of mass dinosaur fossil sites near volcanic areas where CO2 pumped out has suffocated them,
… that caught my attention, can’t see how I missed it when it was first published. Not really my subject, but something that significant, etc.
I suppose there’s no reason why there couldn’t be any co2 lagerstatten thoretically caused by Lake outgassing – much like the event at Lake Nyos a few years ago that killed 1700 people
http://en.wikipedia.org/wiki/Lake_Nyos
very specific meteorological and geographical conditions acted to prevent the mixing and dispersion of the co2 – stable atmosphere and co2 trapped in a bowl shaped depression. In geological terms small scale, although obviously devasting for those involved.
Although it killed all those people it wasn’t enough to register on the Mauna Kea CO2 measurements though – so a relatively big event locally it was insignificant globally.
I doubt if there are that many lagerstatten where co2 poisioning has been the primary kill mechanism though.
Disclaimer…I’m not a paleontologist…one may be along in a bit and if there is one..they’ll know far more about it than me.
DrJFull MemberPosted 10 years agoAs a side comment, a while ago I did some simple maths on how much CO2 would be generated from a full tank in my little Yaris and was quite surprised.
On the assumptions that the fuel was 100% octane and that it burned completely to give carbon dioxide and water it was over 100kg per 45 litre tank.
Makes you think, when you consider how many cars are on the road, for a start, and then the power stations etc.
We’ll in round numbers I think emissions per person in European countries are about 10 metric tonnes, so 10,000 kg or 100 tanks using your calculations. Does that make sense?0
gwaelodFree MemberPosted 10 years agooxygen levels in prehistory are fascinating…increasing oxygen levels allow insects to grow larger – their size is controlled by the rate at which oxygen can diffuse through their body. as oxygen levels in the atmosphere cross certain thresholds though biomass will burn uncontrollably.
during carboniferous period oxygen levels were 30% or so of the atmosphere – insects were mahoosive
bigger than cars they were (although I’m not a paleontologist)
AdamWFree MemberPosted 10 years agoDrJ – I guess so. I try not to drive everywhere as I have bikes. I have a small-sized Yaris. For my car this would mean filling twice a week which I don’t do – once every three or four weeks for me. But it is a 998cc car. For a larger car it would depend on fuel efficiency.
And there are basic assumptions in my calculations: 100% conversion, density of octane at 0.703g/ml and assumption of 100% octane fuel. Burning is rarely 100% efficient and you’ll end up with benzenes etc.
Maths is easy though based upon number of moles of octane per litre multiplied by eight to give number of moles of carbon dioxide multiplied by weight of CO2.
BigEaredBikerFree MemberPosted 10 years ago– although another big slug of CO2 has also found it’s way into the oceans as well in recent years – ( a tiny amount compared with the size and chemical composition of the oceans, but enough to shift the alkalinity slightly)
Can someone brainer than I do some maths using Henry’s law to figure out what the PPM of CO2 in the atmosphere would have to be to actual shift sea water from being alkaline to neutral and then to acid? If I remember my GCSE & AS levels properly it would have to be far more than the current 400ppm given the CO2 that enters the ocean will always maintain proportion with the atmosphere.
Not disagreeing that a very small change in ocean PH won’t cause problems for coral reefs and shell fish etc. which is very frightening (whole food chains could collapse – if we haven’t eaten it all by then) but I know the mass media stories about acidic oceans can’t be true i.e. the oceans will not turn acidic – to get from a PH of 8.10 to less than 7 (neutral) would be mental.
This is a good graph to start off with though.
ernie_lynchFree MemberPosted 10 years agoI don’t know Henry’s Law but I do know that it’s a lot more complex than the amount of carbon dioxide which gets dissolved. The pH of seawater is heavily effected by the natural buffering effect of dissolved salts, it’s not simply the effect of the presence of carbon dioxide.
EDIT : Not just dissolved salts but also undissolved stuff. For example if seawater became more acidic then the White Cliffs of Dover would start to dissolve/dissolve more rapidly, this would to an extent have a buffering effect.
The problem is that the oceans are very stable environments compared to other areas of the world, the life that lives within them have evolved over hundreds of thousands of years in this relatively stable environment and simply can’t make the adjustments that slight change might bring.
The topic ‘Where's all the CO2’ is closed to new replies.