Home › Forums › Chat Forum › Gadget to stop wasting camping gas near empty cans
- This topic has 133 replies, 26 voices, and was last updated 5 years ago by tjagain.
-
Gadget to stop wasting camping gas near empty cans
-
gonefishinFree MemberPosted 5 years ago
Once again – as you attach and detach the can from burners many many times there clearly is no limit to how often the valve can be opened and closed mechanically.
In this instance you are using the system in an approved way as it was designed to be used. By refilling gas bottles in the manner you describe you are doing something to the system that it was not designed for with equipment that has been bodged together. You want to know what can go wrong? Most likely a failure of the system during the filling process that will lead to a projectile type injury. The pressure in a 70/30 mix of Butane Propane will likely be 2.5 – 3.0 barg not massive but it certainly has the potential to go wrong and all for what to save a few pence?
tjagainFull MemberPosted 5 years agoYou want to know what can go wrong? Most likely a failure of the system during the filling process that will lead to a projectile type injury.
really – what would be the mechanism of that failure? Remeber that you attach and detach teh cans from burners many times. Remeber that the burner has exatly the same thread and valve opening tube asthe transfer gadget. So why is this catestrophic failurte more likely with a refil than with a attaching of the burner?
outofbreathFree MemberPosted 5 years agoYou want to know what can go wrong? Most likely a failure of the system during the filling process that will lead to a projectile type injury. The pressure in a 70/30 mix of Butane Propane will likely be 2.5 – 3.0 barg not massive but it certainly has the potential to go wrong
If that were likely then the same risk would apply attaching a stove.
I think doing it 2 or 3 times to a canister that is good condition is probably fine
Me too. And probably a good few other times, as well. Using and refilling gas is *way* safer than pouring meths into a burner and setting fire to it.
GreybeardFree MemberPosted 5 years agoFirst, I do have one of these devices and I think it’s safe if you know what is happening.
I don’t think the reason to be concerned about overfilling has been mentioned yet. It’s the expansion of the liquid, not the gas:
As long as there is a gaseous headspace, the pressure in the gas is the vapour pressure at whatever temperature the gas is at. If the cylinder warms, the gas pressure goes up, but the cylinder is designed for that. The liquid butane (or propane/butane mix) also expands, and it expands a lot. Liquid butane expands 9 times more than liquid water, liquid propane 20 times as much. As the liquid expands it increases the pressure in the headspace so some gas condenses into the liquid. If the cylinder has more than the design weight of gas, when the temperature increases you end up with a cylinder full of liquid, which is incompressible and either ruptures the valve or bursts the cylinder, resulting in release of the entire contents, rapidly.
No it is not pushing thru from gravity – it is the pressure differential. Yes the pressure does equalise between the cvans – its that equalising of pressure that pushes the liquid thru
No, it’s gravity. The liquid in the top cylinder is pulled through the valve by gravity, but the valve doesn’t allow gas bubbles back up. The gas pressure in the top cylinder reduces (you can feel it get cold as liquid evaporates to restore the vapour pressure). The gas pressure in the bottom cylinder increases, and the liquid stops flowing until the gas condenses. It has to lose heat to the environment to do that, which is why the liquid flows fast initially then slows down. Chilling the bottom cylinder first lowers the starting point so that it doesn’t take so long.
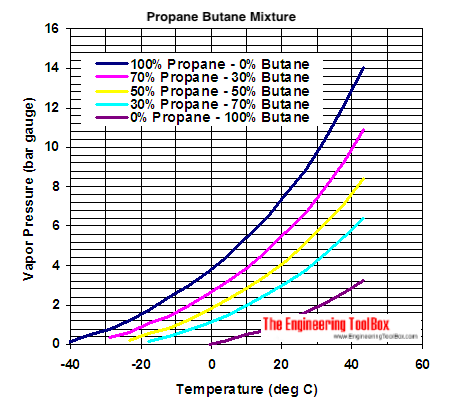
I have a graph of the vapour pressure of various C3/C4 mixes against temperature. I showed this to a friend who thought it would be a good idea to refill C3/C4 mix cylinders with pure propane. That is NOT a good idea. I’ll post that later if I can find it.
martymacFull MemberPosted 5 years agoCommon sense should tell us that the valve in a disposable canister is safe to use, and will be safe to attach/burn/detach multiple times, it will also ‘probably’ be safe to refill and repeat the process a few times.
But common sense should also tell us that the valve isn’t designed to last forever, so there has to be a point where it’s no longer sensible to continue using the same canister, after all, even the heavy duty valves used on industrial cylinders get refurbed every time they go back to the filling depot.outofbreathFree MemberPosted 5 years agoBut common sense should also tell us that the valve isn’t designed to last forever, so there has to be a point where it’s no longer sensible to continue using the same canister
No quarrel with that. I can’t find anywhere that tells me how long it’s safe to store cannisters in terms of time or in terms on number of attachments/detachments. I’d be interested to know.
tjagainFull MemberPosted 5 years agoNo, it’s gravity. The liquid in the top cylinder is pulled through the valve by gravity, but the valve doesn’t allow gas bubbles back up
I am sure this is not right. IME it does not work unless you have a pressure differential and how can an open valve stop gas bubbles. When I do it you can hear the hiss as pressure equalises as it pushes the gas thru.
outofbreathFree MemberPosted 5 years agoI have a graph of the vapour pressure of various C3/C4 mixes against temperature.
I showed this to a friend who thought it would be a good idea to refill C3/C4 mix cylinders with pure propane.
Depending on the temperature the cannister was going to experience that would be absolutely fine. (In the real world the risk would be filling for use in a cold snap in midwinter and then forget to empty it before summer, or putting it in a warm car to get to the campsite.)
thisisnotaspoonFree MemberPosted 5 years agoI am sure this is not right. IME it does not work unless you have a pressure differential and how can an open valve stop gas bubbles. When I do it you can hear the hiss as pressure equalises as it pushes the gas thru.
If you have an empty can (as in there’s zero liquid left) then the pressure will drop below the vapour pressure. But upto that point (or once it’s begun filling) then the pressure will be equal to the vapour pressure.
Cooling one cylinder is simply manipulating the vapour pressure.
Greybeard is probably correct, there won’t be any gas transfer, it will rely on the liquid in the upper cylinder vaporizing, and then absorbing heat from the surroundings.
Side note:
The lower cylinder temperature won’t rise significantly (beyond any change due to chilling the cylinder beforehand then the ambient liquid filling it and a very slight change due to the work done pressurising it both offset against the initial portion of the gas vaporizing as the pressure is below it’s vapour pressure). And thermodynamically the volume has increased, therefore liquid has turned to vapour so overall the system must absorb energy.
thisisnotaspoonFree MemberPosted 5 years agoDepending on the temperature the cannister was going to experience that would be absolutely fine. (In the real world the risk would be filling for use in a cold snap in midwinter and then forget to empty it before summer, or putting it in a warm car to get to the campsite.)
Can you explain your thinking on that?
This is what a propane cylinder looks like, it’s A LOT tougher than the pressed metal butane and 70/30 mix cans. Putting propane in a butane or 70/30 mix can is stupid beyond belief!
 GreybeardFree MemberPosted 5 years ago
GreybeardFree MemberPosted 5 years agoI am sure this is not right. IME it does not work unless you have a pressure differential
TJ, there is no pressure differential. The gas pressure is the same, irrespective of the amount of butane. Besides, I use the gadget to drain the dregs of a mostly empty cylinder into a half full one; if there was a pressure differential, it would be the wrong way.
If you don’t understand why the pressure in the gas only depends on temperature, the rest of the discussion will be beyond you.
tjagainFull MemberPosted 5 years agoIf you don’t understand why the pressure in the gas only depends on temperature,
I do understand that ( posted many times) and I have had no success in half full to half full. I always cool the bottom one to ensure a pressure differential and it certainly sounds like its filling under pressure of that differential – Tsssssss
I’ll try it without chilling next time.
outofbreathFree MemberPosted 5 years agoCan you explain your thinking on that?
I posted a link to the graphs.
At low temperatures Propane will be lower pressure than Butane is at high temperatures.
So at low temperatures you can store propane in a canister that’s only designed to withstand butane pressures. See the charts for the graph that tell you how cold the conditions the cannister experiences has to be. (EDIT: I couldn’t resist checking the magic point. If the canister was only seeing temperatures around -10 or lower it would be fine.)
It’s a bit hypothetical, I can’t imagine anyone would do it, or would live/camp in a situation where it would be possible, but you could.
thisisnotaspoonFree MemberPosted 5 years agoThe gas cans we are talking about are butane / propane mix.
If you were filling a mix can from another mix can then Greybeard is even more right, the near empty can will be mostly butane as the propane evaporates first (unless they have a dip tube which i don’t think they do?) which would have a very low vapour pressure compared to a full can of C3/C4 mix.
SpinFree MemberPosted 5 years agoRefilling disposable gas canisters. Very sensible.
Providing you follow some fairly simple rules it works fine.
Do it in a well ventilated area, refill like with like, don’t overfill and don’t refill the same canister more than maybe 3 or 4 times.
I’m happy that this minimizes the risks involved sufficiently. You might not be but that’s your choice.
SpinFree MemberPosted 5 years agoI haven’t read this whole thread so can someone tell me:
Are people suggesting this doesn’t work?
Or are we just arguing over how / why it works?
GreybeardFree MemberPosted 5 years agoOr are we just arguing over how / why it works?
We’re arguing whether it’s safe. And some of us are arguing that it’s only safe if you understand how it works.
thisisnotaspoonFree MemberPosted 5 years agoAt low temperatures Propane will be lower pressure than Butane is at high temperatures.
So at low temperatures you can store propane in a canister that’s only designed to withstand butane pressures. See the charts for the graph that tell you how cold the conditions the cannister experiences has to be. (EDIT: I couldn’t resist checking the magic point. If the canister was only seeing temperatures around -10 or lower it would be fine.)
It’s a bit hypothetical, I can’t imagine anyone would do it, or would live/camp in a situation where it would be possible, but you could.
That Propane cylinder has a design pressure of 22.4bar (presumably barg), which equates to about 70C. At the same temperature butane is about 6.8bara (5.8 barg), so in the absence of my google fu failing me, that would be a sensible design pressure for the butane cylinder, assuming Rault’s Law and ideal liquids (reasonable, both are low molecular weight hydrocarbons) that would give a 70/30 mix can a design pressure of 10.8barg.
That corresponds to a temperature of a little over 30C.
30C is nothing, that’s a gas canister exploding* if someone put’s it in their sleeping bag to keep it warm overnight. Or the boot of your car on a warm day in February. What’s the temperature inside the windshield around the stove?
You’re taking something with a design pressure that’s never likely to be exceeded anywhere outside of the desert, and reducing that to be careful not to warm it up with body heat.
Considering you can buy Propane cylinders, trying to fill a butane or mix cylinder with propane is just monumental stupidity when propane cylinders exist.
*presumably the valve is designed to vent and/or fail first so leaking is maybe a better description.
RockhopperFree MemberPosted 5 years agoI’ve just ordered one of those valves but why are we suggesting its safe only if we do it three of four times? The valve on the canister can handle many more cycles than that and I’d suggest that the cans themselves are over engineered to handle the kind of use that camping might expose them to without failure.
outofbreathFree MemberPosted 5 years agothe near empty can will be mostly butane as the propane evaporates first
There was some doubt about that theory expressed earlier in this thread. Not sure we came a conclusion we all agreed on. If we have I’d be interested to know.
outofbreathFree MemberPosted 5 years agoThat Propane cylinder has a design pressure of 22.4bar (presumably barg), which equates to about 70C. At the same temperature butane is about 6.8bara (5.8 barg), so in the absence of my google fu failing me, that would be a sensible design pressure for the butane cylinder, assuming Rault’s Law and ideal liquids (reasonable, both are low molecular weight hydrocarbons) that would give a 70/30 mix can a design pressure of 10.8barg.
That corresponds to a temperature of a little over 30C.
30C is nothing, that’s a gas canister exploding* if someone put’s it in their sleeping bag to keep it warm overnight. Or the boot of your car on a warm day in February. What’s the temperature inside the windshield around the stove?
You’re taking something with a design pressure that’s never likely to be exceeded anywhere outside of the desert, and reducing that to be careful not to warm it up with body heat.
Considering you can buy Propane cylinders, trying to fill a butane or mix cylinder with propane is just monumental stupidity when propane cylinders exist.
I can’t see the relevance of this to what I wrote.
thisisnotaspoonFree MemberPosted 5 years agoI can’t see the relevance of this to what I wrote.
The bit where you advised putting propane into canisters not designed to hold propane.
outofbreathFree MemberPosted 5 years agoI’ve just ordered one of those valves but why are we suggesting its safe only if we do it three of four times?
Not sure we are. I think we’re saying it *is* safe if you only do it three or four times. Not that it’s unsafe if you do it more.
I kept a Coleman cannister for two years and must have refilled it a multiple of 4 times. It was used around salt water and had rust specs on the outside. I got rid of it because of it’s age not because of the amount of use it had had.
As you say, the cannisters must be designed to take a hell of a bashing. (I hadn’t considered that.)
The risk that’s been highlighted is the risk of an adapter/stove ‘popping’ off and smacking the user in the eye. I think that’s laughable.
outofbreathFree MemberPosted 5 years agoThe bit where you advised putting propane into canisters not designed to hold propane.
Cite.
crimsondynamoFree MemberPosted 5 years agowhy are we suggesting its safe only if we do it three of four times? The valve on the canister
Because the canister will have been designed accounting for the fact that it will be pressurised once, and the valve will undergo a reasonable number of clicks corresponding to one canister. That design will then have a massive safety margin built-in, so that on the bell curve of probability an brand new can/valve will only fail say (for the sake of argument) once in a billion. As you exceed those design parameters, you start creeping along the bell curve. One in a billion becomes one in a hundred million. Then curve steepens and the odds reduce drastically.
So basically if you know what you’re doing you can reuse a few times and you’re only creeping along the valley floor of the bell curve.
GreybeardFree MemberPosted 5 years agoI always cool the bottom one to ensure a pressure differential and it certainly sounds like its filling under pressure of that differential – Tsssssss
I’ll try it without chilling next time.
OK, I see why you think pressure is driving it. It does work without chilling, but the flow rate slows down sooner. The pressure differential will drive it initially if there’s a temperature difference, but after that it’s gravity and waiting for the top cylinder to gain heat while the bottom one looses it. Unless I have more weight in the top cylinder than I want to transfer, I just leave it all connected for a hour or two, outside, and the top cylinder will empty completely. You can hear the liquid dripping through.
The valve tube is only small diameter and surface tension in the liquid is enough to stop gas bubbles rising through it.
outofbreathFree MemberPosted 5 years agoBecause the canister will have been designed accounting for the fact that it will be pressurised once
Ehhh? At no point in the use/refilling of a gas canister does it become de-pressurized unless you 100pc empty it which in practice never happens. Certainly I’ve never filled a cannister with 0 fluid in it. So the pressure never changes from the pressure experienced by any other canister. It will just wander around with temperature.
and the valve will undergo a reasonable number of clicks corresponding to one canister.
Yup, and nobody can find what that number is. Which makes me think it’s so astronomical it’s not worth thinking about otherwise Coleman would stamp <Warning: Don’t not connect to a stove more than 500 times> on the can. And they don’t.
thisisnotaspoonFree MemberPosted 5 years agoCite.
Depending on the temperature the cannister was going to experience that would be absolutely fine.
I just showed that your assertion that putting propane in a different canister was safe “depending on temperature” was very much not safe as you could exceed the design pressure of the can even under ambient UK temperatures or doing relatively normal things with a gas canister in winter.
GreybeardFree MemberPosted 5 years agothe near empty can will be mostly butane as the propane evaporates first
There was some doubt about that theory expressed earlier in this thread. Not sure we came a conclusion we all agreed on. If we have I’d be interested to know.
I don’t think we did, but I tend to view that the mix proportions change a bit but not a lot. I should be doing something else so haven’t read the following link fully, but it looks useful.
https://bushwalkingnsw.org.au/clubsites/FAQ/FAQ_Mixtures.htm
outofbreathFree MemberPosted 5 years agoThe bit where you advised putting propane into canisters not designed to hold propane.
!=
Depending on the temperature the cannister was going to experience that would be absolutely fine.
What I said was a) Correct. b) Not Advice.
I won’t be replying to you again so you’ll have the joy of the last word.
I just showed that your assertion that putting propane in a different canister was safe “depending on temperature” was very much not safe as you could exceed the design pressure of the can even under ambient UK temperatures or doing relatively normal things with a gas canister in winter.
-10C is not a UK ambient temperature. (Unless you live in my old student flat in which case you have a point.)
outofbreathFree MemberPosted 5 years agoI don’t think we did, but I tend to view that the mix proportions change a bit but not a lot.
The two theories seem to be 1) propane evaporates first and 2) empty-ish cans cool faster.
2) Is definitely true. I’d love to know if 1) is as well.
Apropos of nothing:
 gonefishinFree MemberPosted 5 years ago
gonefishinFree MemberPosted 5 years ago1) propane evaporates first
If your thinking is that all the propane boils first and then the butane boils as individual components then the answer is no. The vapour will initially be relatively richer in propane but the compositions of both the liquid and gas will vary as gas is removed, assuming that the mixture is not azeotropic.
The composition of the gas in equilibrium above a mixture, and the way those compositions vary as vapour is removed, is more complex than can realistically be discussed on an internet forum. Even for a relatively simple binary mixture of propane and butane.
crimsondynamoFree MemberPosted 5 years agounless you 100pc empty it which in practice never happens.
You cannot speak for everyone’s filling/refilling regime. For example:
I just leave it all connected for a hour or two, outside, and the top cylinder will empty completely.
crimsondynamoFree MemberPosted 5 years ago<Warning: Don’t not connect to a stove more than 500 times> on the can. And they don’t.
Of course they don’t. It would be ridiculous to suggest that a valve can be engineered to be fine on the 500th connection but fail on the 501st.
What can be engineered is for a valve to have a miniscule failure rate to start with. However as you make more and more connections the odds start changing. No one of us knows what the curve looks like, but the odds do change.
outofbreathFree MemberPosted 5 years agounless you 100pc empty it which in practice never happens.
You cannot speak for everyone’s filling/refilling regime.
I think I can. Once the can reaches 1atm no more gas escapes. …but that doesn’t matter because the cans must be designed to handle a pressure of 1atm multiple times because the gas itself can reach 1atm. So being completely de pressurized is a normal situation, even in an unused cannister straight out of the factory.
outofbreathFree MemberPosted 5 years agoIf your thinking is that all the propane boils first and then the butane boils as individual components then the answer is no. The vapour will initially be relatively richer in propane but the compositions of both the liquid and gas will vary as gas is removed, assuming that the mixture is not azeotropic.
The composition of the gas in equilibrium above a mixture, and the way those compositions vary as vapour is removed, is more complex than can realistically be discussed on an internet forum. Even for a relatively simple binary mixture of propane and butane.
I was quoting someone else but to be clear what I (and others) have been asking is whether a cannister of 30/70 propane butane that’s had a lot of contents used on a stove/whatever is still at the 30/70 ratio.
thisisnotaspoonFree MemberPosted 5 years agoAnything cooler than -1C would result in an almost complete vacuum even when the can is “full”. Not the same as “100pc empty”, but does nicely illustrate the point that the mass of fuel in the canister isn’t related to the pressure (as long as it’s in an equilibrium with a vapour).
I was quoting someone else but to be clear what I (and others) have been asking is whether a cannister of 30/70 propane butane that’s had a lot of contents used on a stove/whatever is still at the 30/70 ratio.
It will vary depending on the temperature when it’s used.
At 30C (i.e. summer) a can containing a 70/30 mix would have a vapour consisting of about 3 parts butane 10.75 parts propane (those being the vapour pressures in bara at 30C).
At -10C the only (to within a few decimal places, there will be butane but only a very small amount) component in the vapour space is Propane (at about 3.75bara).
Unless a mixture has an azeotrope (a point in the distillation curve where the vapour composition matches the liquid composition so neither can change) then one will always boil off before the other. A practical example is ethanol, you can’t distil past 95%, to get purer alcohol than that you have to distil it to 95% then strip the water some other way e.g. by adsorbing it onto a dessicant. Or you absorb the alcohol into something else (a high molecular weight hydrocarbon like diesel might work for example), then distil the diesel/ethanol mixture.
The topic ‘Gadget to stop wasting camping gas near empty cans’ is closed to new replies.
